It is well known that antibiotics are used as a widespread cure for bacterial infection. In fact, according to the Centers for Disease Control and Prevention (CDC), as of 2022, 236.4 million antibiotics were prescribed by healthcare providers in the U.S..
Before the rise of antibiotics, the global average life expectancy was around 47 years. This number has now risen to a sizable 78.8 years. While antibiotics have significantly hindered the prevalence of bacterial infection, it is still an issue in the modern day. Natural selection favors bacteria that can resist antibiotics, which can cause a problem known as antimicrobial resistance (AMR) that killed around 1.27 million people globally in 2019, 60,000 of which were in the United States.
AMR arises in two main scenarios: when an antibiotic is not taken for its full dosage period, allowing the stronger bacteria to survive and come back stronger, and when a prescribed antibiotic is more powerful than what is necessary, resulting in bacteria that have a high resistance to antibiotics.
These issues can be countered simply by taking antibiotics for the full dosage period and prescribing medication as needed. Instead of the most powerful agent to kill bacteria, doctors can prescribe a medication that is just enough to purge the infection, which slows the rate of AMR, and in most cases, stops it altogether as all the bacteria are eliminated. This allows doctors to save just as many lives, while preventing bacteria from becoming resistant to medications. However, it is easier said than done.
Third world countries highly benefited from the invention of antibiotics, but their distribution to these countries became a problem. The use of antibiotics is unregulated, and oftentimes people take the drugs incorrectly or worse, take the incorrect drugs. When potent antibiotics are given to people that do not need them, the bacteria that end up surviving are resistant to even the most powerful antibiotics. This creates the problem of antimicrobial resistant bacteria that can spread across the globe.
However, developing countries are not the only ones that create this problem; medicines in more developed countries also struggle against these extremely resistant bacteria.The CDC reports that, “at least 28% of antibiotics prescriptions are unnecessary in U.S doctor’s offices and emergency departments.”
For example, vancomycin, widely known as a “last resort” antibiotic that can kill many bacteria resistant to weaker antibiotics, is becoming increasingly ineffective against resistant bacteria. These powerful microorganisms, such as vancomycin-resistant enterococci and vancomycin-resistant Staphylococcus aureus, are the strongest survivors of incomplete or hasty exposure to vancomycin.
Fortunately, there are methods available to treat these antimicrobial resistant bacteria due to the creation of new, stronger antibiotics and the production of analog forms of vancomycin that greatly improve efficiency. Still, the question remains of what to do once we run out of alternatives.
There is an inherent need for action against AMR in the United States, but no major actions are currently being implemented. In the last 20 years, the U.S. Department of Agriculture has only approved two new classes of antibiotics against gram-positive bacteria that have little effect on gram-negative bacteria. Without change, the number of deaths due to AMR will continue to rise.
The solution of engineering new drugs to treat AMR is limited by the fact that we may reach a limit in antibiotic strength.. This is why modern medicine is exploring new options to combat AMR beyond antibiotics, and one option being explored comes from an unexpected ally and one of bacteria’s greatest enemies: bacteriophages.
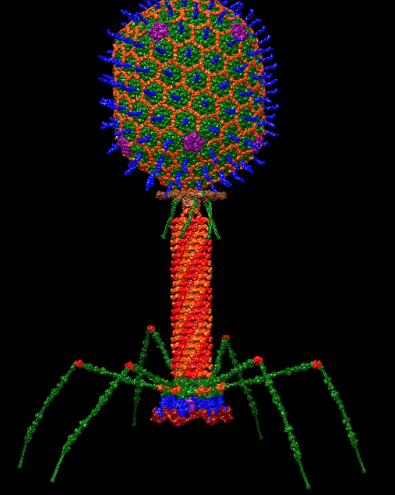
The bacteriophage, a type of virus that specifically targets bacteria, is a specialist in killing hardy bacteria. Simply known as phages, they target exclusively bacteria and prove no harm to human cells. Because they are not alive, there is little concern of ethical issues. Phages can also be altered to target a specific type of bacteria.
Phage therapy is not something new; it has existed since the early 1900s, but generally has been overshadowed by antibiotics. Bacteriophages also fix many of the problems associated with the overuse of antibiotics in various industries. By using bacteriophages in the agricultural industry, antibiotics do not have to be used on livestock in excess. This also helps to reduce the prevalence of AMR.
Phages can be modified to target only specific forms of bacteria so that they do not harm the “good” bacteria that helps the human body function. Due to the fact that bacteriophages do not attack bacteria in the same way that antibiotics do, even the most antibiotic resistant bacteria will still be susceptible to phages.
However, there are some drawbacks. Phages have not been researched enough to know if they are truly safe for human cells. While they do not directly attack human cells, they can cause endotoxins from the bacterial cell wall to be released into the body when attacking bacteria, which can lead to other forms of infection and damage.
Phages are also not a universal solution, as they need to be manufactured in a specific way to target certain bacteria. Bacteria can also develop resistance to bacteriophages just as human cells do to viruses. Even though phages can also evolve, more research needs to be done on how to make this practical and efficient. Additionally, while bacteria resistant to phages can have a discovered weakness to antibiotics, some phage-resistant bacteria simply become more virulent.
It is not very probable that bacteriophages will become as universal a solution to bacterial infection as antibiotics, and more research needs to be done to see if bacteriophages can be utilized to target specific bacteria. Even so, it is an innovative solution that could have great value in the world of epidemiology.
Though the issue of AMR as a whole is related to the inconsistency of antibiotic availability in third world countries, it is still prevalent even in developed countries like the United States. According to the National Library of Medicine, many Americans are not well informed about antibiotics, as about 53 percent of Americans incorrectly stated that antibiotics are effective against viruses, and that “patients often do not understand how to take antibiotics as they are prescribed.”
Apart from supporting continued research into new solutions for AMR, people can combat the issue of antimicrobial resistance by spreading awareness and educating others on how to correctly use antibiotics and what they are effective for. When people know how to take antibiotics, they can prevent AMR by fully cleansing the body of harmful bacteria instead of leaving highly-resistant bacteria.